



The Roles of Plating
The role of plating, in a nutshell,
is to add value by giving new functions to the surface of objects.
Let’s learn about what functions plating provides.
What functions does plating provide?
One characteristic of plating is its ability to impart various features depending on the type of metal and the process used.
Let's take a look at some of the most common functions and examples of their use.


Decorative plating
Plating is not only used to give esthetic beauty and a sense of luxury to accessories, furnishings, and the interiors and exteriors of vehicles; weight reduction is another major objective. Decorative plating includes plating with precious metals such as gold, silver, rhodium, and chrome (with an undercoat of copper or nickel plating).
Anti-corrosion plating to prevent rust
This type of plating is primarily used on iron and steel materials for anti-corrosion purposes (to prevent rust). Its role is to protect iron surfaces so that, even if oxygen or chlorine in the atmosphere or water adheres to the surface, corrosion does not penetrate into the material. Zinc-plating and tin-plating are common examples of anti-corrosion plating.


Hard plating to prevent wear
Because chromium is an extremely hard material with excellent abrasion resistance, it is used for hard chrome plating, in which a thick film is plated to the sliding parts of mechanical components in automobiles, motorcycles, aircraft, etc. Electroless nickel plating is also used to enhance wear resistance.
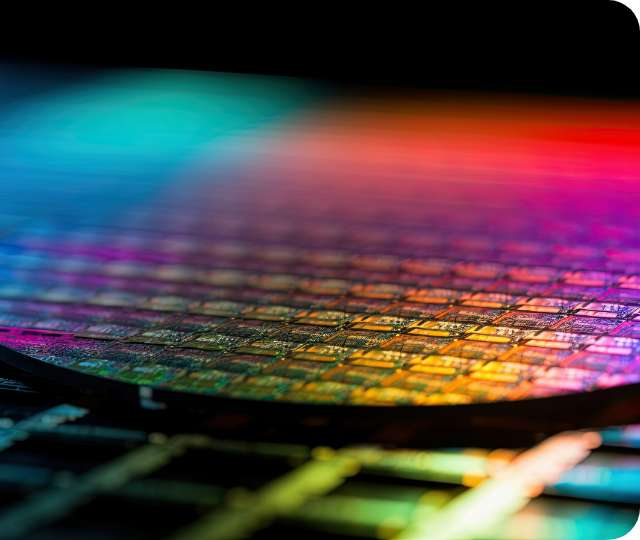
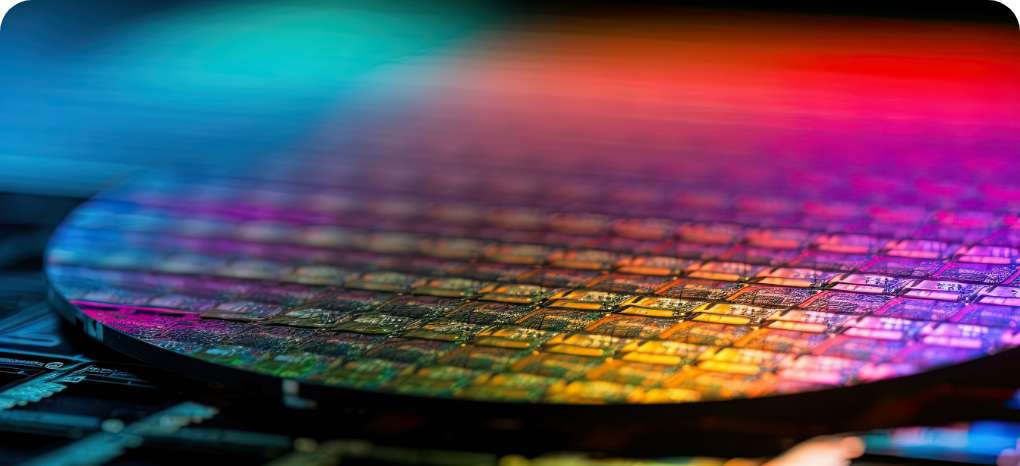
Plating for circuit formation
This type of plating is used to conduct electricity through wiring and connections in electronic components. Copper plating in particular, is used because it is one of the most conductive metals (easy to pass electricity through it).

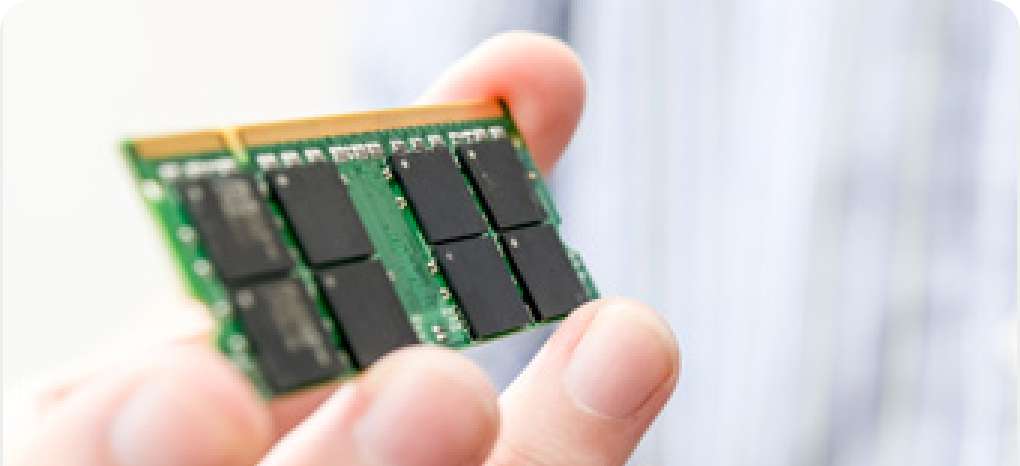
Plating for circuit connection
Gold, silver, copper, nickel, tin, and other plating can provide a bonding surface suitable for a variety of connection methods, including contact, solder, wire, and sintered materials.


Plating that imparts magnetic properties
This type of plating is used on storage media such as hard disk drives and on PC and smartphones cases. Plating that imparts magnetic properties includes cobalt alloy plating, while electroless nickel plating is one method of plating used to block the effects of magnetism.

What metals can be used for plating?
Plating is a technique in which a metal is deposited on the surface of an object by reducing metal ions contained in a chemical solution.
In this section, we will learn about the properties and the roles of different plating metals (coating materials).
Gold-plating
Gold, the most popular precious metal used for plating, has low electrical resistance and excellent durability. It plays a wide range of roles and is often used on contact points and terminals in electronic components.

Silver-plating
Silver is not only pleasing to the eye, but provides an antibacterial effect. It is therefore used on things that we often touch, such as tableware. It is also frequently used in electronic components because of its low electrical resistance.

Copper-plating
Copper has excellent conductivity and is often used for the wiring of electronic circuit boards. Because of the relative ease of plating with copper, it is often used as an undercoat.

Tin-plating
Tin-plating has been used since long ago to prevent rust. It is still often used today for such applications as electronic component connections.

Nickel-plating
Nickel is relatively corrosion-resistant and has excellent mechanical properties, so it is used in a wide variety of applications. This includes imparting hardness to the surface, making the surface flatter and less resistant, and preventing magnetic waves in electronic devices.
Connection properties Heat resistance properties Magnetic properties Anti-rust

Chrome-plating
Chrome-plating is often used for rust prevention and decorative purposes. It is used on hard surfaces, and on such parts as shafts, valves, piston rings, and axle bearings. Chrome-plating is also used for molds, due to its non-adhesiveness.

Zinc-plating
Zinc-plating has been used as anti-rust plating for a long time. One common example is the hot dipping method known as "hot-dip galvanization." As a low-cost means to prevent rust on iron, it is still used in large structures, bolts, nuts, aerial wiring fittings, and other parts.

Composite-plating
Composite-plating is a kind of plating in which insoluble fine particles are dispersed in the plating solution and made to undergo an eutectoid reaction with the plating film, thereby imparting new functions that cannot be obtained with existing films. For example, combining PTFE (polytetrafluoroethylene, a resin formed from fluorine and carbon) and nickel in a eutectoid alloy can greatly decrease the surface friction of the resultant substance, and can be used in applications that require water repellency or smooth machine component movement.


The roles of plating presented in this chapter are representative examples, and there are many more applications to be found. In this chapter, we have learned mostly about single-metal plating. However, combining two or more metals to create alloy plating can give the film even more diverse functions. We are still mid-journey in the development of plating applications, and it has the potential to create new technologies, depending on the combination of metals used for plating and where plating is used.

